

Laboratory diagnosis of intestinal parasitic infections can be carried out by detection and identification of the parasites or their particular stages (ova/egg, cyst, larva or trophozoite) in the stool specimen.
Stool sample collection:
Stool specimen should be collected in a wide-mouthed, clean, leak-proof container. The Sample should be collected after onset of symptoms and ideally before the initiation of the antiparasitic therapy. First morning sample is preferable. The amount of stool and the frequency of stool specimen submission differ according to the suspected disease. Generally collections of three stool specimen is sufficient to make diagnosis of most intestinal parasitic infections but for some diseases like Giardiasis or Amoebiasis a total of six specimens is preferable.
Transport of stool specimen:
Liquid & semi formed stool specimen should be examined within 30 minutes of collections to visualize motility of trophozoites (if Giardiasis/Amoebiasis is suspected). Further delay in examination may disintegrate the trophozoites. Formed stools may be examined up to 24 hours after passage.
Intestinal Parasites found in Feces:
|
Name of the Parasite |
Protozoa or Helminth |
Disease |
|
Protozoa |
Amoebiasis & Ameobic liver abscess |
|
|
Protozoa |
Giardiasis |
|
|
Cryptosporidium parvum |
Protozoa |
Cryptosporidiosesis |
|
Cystoisospora belli, (Previously known as Isospora belli) |
Protozoa |
Cystoisosporiasis |
|
Cyclospora cayetanensis |
Protozoa |
Cyclosporiasis |
|
Balantidium coli |
Protozoa |
Balantidiasis |
|
Taenia solium (pork tapeworm) |
Helminth |
Taeniasis & Cysticercosis |
|
Taenia saginata (beef tapeworm) |
Helminth |
Taeniasis & Cysticercosis |
|
Hymenolepis nana (dwarf tapeworm) |
Helminth |
Hymenolepiasis |
|
Schistosoma mansoni, S. haematobium, S. japonicum |
Helminth |
Schistosomiasis |
|
Fasciola hepatica (the common liver fluke) or or (the sheep liver fluke). |
Helminth |
Fascioliasis |
|
Ascaris lumbricoides (large roundworm) |
Helminth |
Ascaris (Ascariasis) |
|
Ancylostoma duodenale (Old World hookworm) |
Helminth |
Hookworm infection |
|
Necatar americanus (New World hookworm) |
Helminth |
Necatoriasis (Hookworm infestations) |
|
Enterobius vermicularis (Pin worm) |
Helminth |
Enterobiasis |
|
Trichuris trichiura (Whip worm) |
Helminth |
Trichuriasis (Whipworm Infection) |
Preservation of the stool specimen:
Preservatives (like formalin, Polyvinyl alcohol) are being used for the permanent fixation of stool specimens. Preservatives kill the parasites so characteristics motility of the trophozoites cannot be seen. Preservatives helps to preserve morphological forms (cyst, ova, and larvae) of parasites while being sent to reference laboratories for analysis and also lowers the risk of infections to the laboratory technicians.
Laboratory detection methods for Intestinal Parasitic Infections
Macroscopic examination:
Stool sample is first examined macroscopically for its consistency (formed, semi-formed or liquid), colour, odour and presence of blood or mucus. Occasionally adult intestinal helminths e.g. Ascaris lumbricoides and Enterobius vermicularis or segment (proglottids) of tapeworms may be seen in the stool specimen. Presence of blood and mucus in stool may be because of amoebic dysentery or invasive infections such as balantidisis, schistomiasis or in severe infections with Trichuris trichiura. In giardiasis, the stool specimen may be fat-colored and frothy.
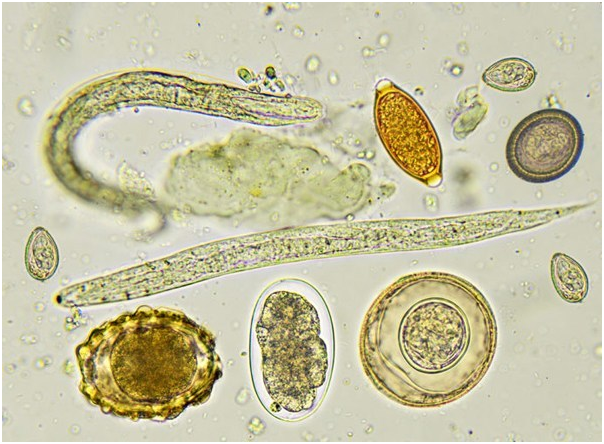
Eggs (ova) of various intestinal parasites
Microscopic examination of the stool is done to detect and identify the cyst and/or trophozoites of protozoan parasites. In the case of helminthic infestations eggs (ova), larvae or segments of helminths can be seen. Direct wet mount (saline or iodine) of stool specimen is routinely done in diagnostic laboratories for the detection and identification of agents of intestinal parasitic infections. To increase the detection rate (sensitivity), stool specimen can be concentrated by various flotation/sedimentation techniques. Smear from the concentrated sample (e.g. sediment) or permanent stained smear (e.g. by Iron-hematoxylin stain, Trichrome stain, Modified acid-fast stain) are used as diagnostic tool depending on the suspected infections.
Culture Methods:
Unlike Bacteriology, culture methods are rarely used as a diagnostic tool in parasitology. Culture methods are available for some of the protozoan parasites (Entamoeba histolytica, Balantidium coli), and Helminths e.g. Harada-Mori culture for recovering larvae of nematodes such as Hookworms, Strongyloides stercoralis.
Serology:
When routine Ova and Parasite Exam (O & P exam) fails to identify the agents responsible for a suspected parasitic infection or when invasive parasitic infection is suspected, serological test are used to make diagnosis. Serological tests are available for the diagnosis of amoebiasis, giardiasis, cysticercosis, schistosomiasis, strongyloidiasis and toxoplasmosis. Direct fluorescent antibody (DFA) assay, Enzyme immunoassay (EIA) and Rapid immunochromatographic cartridge assays are commonly used serological techniques.
Molecular Assay:
Molecular assays (e.g. nucleic acid amplification tests such as PCR, DNA Probes) are reliable but expensive diagnostic tools which can be used for the diagnosis of intestinal parasitic infections. So far, molecular assays are not preferred method of diagnosis for stool parasites.
